6, Aug 2024
The World’s largest cancer awareness run Grace Cancer Run 2024 will be held in October 2024
Hyderabad, August 6 , 2024 – The World’s largest cancer awareness run Quambiant Grace Cancer Run 2024 will be held in October 2024. The main aim of the run is to raise awareness and funds for cancer research and screening.
It is being organised in partnership with 6HYSEA, SCSC (Society for Cyberabad Security Council) and many other organisations
The run is to be held on 6th October, with more than 1 lakh participants from across 130 countries through Physical and Virtual Modes expected to participate.
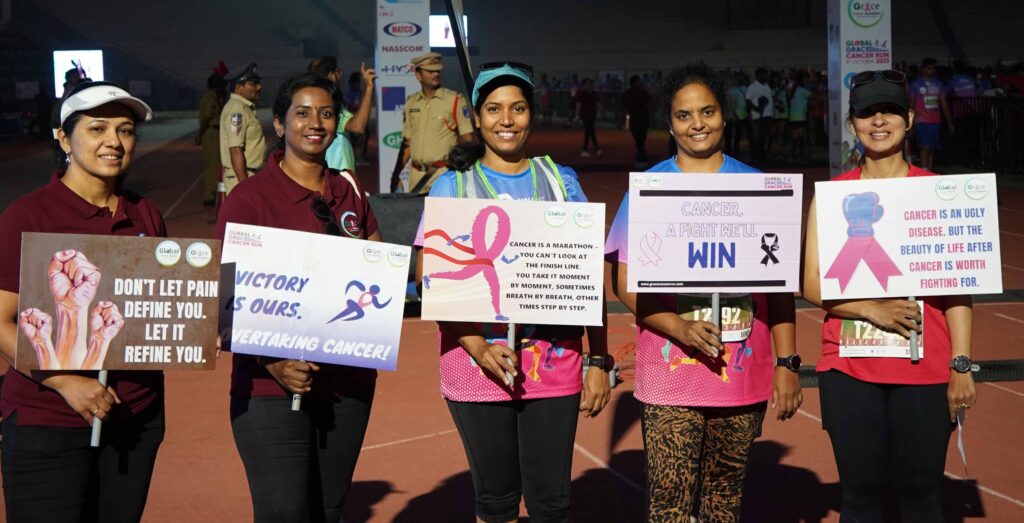
This year’s theme, “Run for Grace Screen for Life,” highlights the importance of early detection and preventive measures in the fight against cancer.
The Race Categories are 2k Walk, 5k Fun Run & 10k (timed run). It will be held at Gachibowli Stadium, Hyderabad. For registrations, please visit the website – www.gracecancerrun.com and register online.
The participation in the run underscores the significance of community involvement and support in our collective battle against cancer said Dr. Chinnababu Sunkavalli, Senior Consultant Robotic Surgical Oncologist and the Founder of GRACE (Global Research and Cancer Education) Cancer Foundation
Grace Cancer Run is more than just a race; it is a movement dedicated to saving lives through awareness, education, and early detection. By participating, you contribute to vital cancer screening programs and research initiatives, he added.
We want to see a world where there is no cancer. There are four health problems responsible for nearly 80% of deaths in India. And they are Cancer, Heart, Lung and Diabetes, he said. With the funds generated from the Run, we have screened 40,000 poor, rural populations. This year we aim to screen one lakh people free of cost
- 0
- By Rabindra
3, Aug 2024
SPARSH Hospital Launches New Back Pain Clinic on International Self Care Day
Bengaluru, 3rdAug 2024 – On this International Self Care Day, SPARSH Hospital launched its new Back Pain Clinic, a comprehensive wellness program designed to address the increasing prevalence of back pain.
The clinic was inaugurated by Dr. Sharan Shivaraj Patil, Chairman & Chief Orthopaedic Surgeon, SPARSH Group of Hospitals, Bengaluru, and Dr. Naveen S Tahasildar, Consultant Spine Surgeon, SPARSH Hospital, Infantry Road.
Dr. Naveen S Tahasildar highlighted the importance of holistic care in managing and preventing physical impairments, particularly back pain. “Once patients overcome their initial issues, maintaining their health through lifestyle modifications and exercises is crucial. To support this, the clinic has introduced wellness programs like yoga and dance movement therapy. Self-care is essential, not selfish, and addressing postural imbalances through physiotherapy is key. Patients learn proper ergonomics and exercises to prevent issues from recurring. The goal is to transition from being a patient to a person focused on self-care, ensuring long-term well-being.”
The Back Pain Clinic aims to tackle the root causes of back pain, which are often linked to sedentary lifestyle, long working hours, and poor posture. This initiative is timely, as back pain is becoming increasingly common among younger individuals and corporate employees.
Mr. Joseph Pasangha, COO of SPARSH Group of Hospitals, Bengaluru, emphasized the significance of this launch: “Our new Back Pain Clinic is a step towards providing holistic and preventive care. We recognize the challenges posed by modern work environments and are committed to offering solutions that promote overall health and wellness. This clinic is not just about treating pain but about fostering a healthier lifestyle for people.”
The clinic offers various therapies, including dance therapy, yoga therapy, and fitness therapy, with the aim of promoting a pain-free lifestyle.
2, Aug 2024
India’s First TAVR Procedure Performed on Lung Transplant Patient for Severe Aortic Stenosis

Dr. Ravinder Singh Rao, Interventional Structural Cardiologist and TAVR expert.
Mumbai, 2024: Medical intervention can be life-saving in dire situations. A timely and targeted intervention can add years to a patient’s life. This has never been highlighted more than in the following case of a 72-year-old woman.
The patient underwent a lung transplant surgery five years ago for severe lung disease. Recently, she faced symptoms of shortness of breath on minimal exertion, which was then diagnosed as severe aortic stenosis. There is no medicine for aortic stenosis, and in very severe cases requires a valve replacement. However, due to her prior transplant, open-heart surgery was not an elective or emergency option. She was referred to Dr Ravinder Singh Rao, Interventional Structural Cardiologist and TAVR specialist. The TAVR, or Trans-Aortic Valve Repair surgery, is a non-invasive alternative to a valve replacement. The patient underwent a CT angiography so Dr Rao could accurately plan the procedure by understanding the size of the valve and artery. This also helped the doctor understand the risk assessment of the procedure.
Severe aortic stenosis refers to the narrowing of the aortic valve, which is the heart’s main valve and controls blood flow into the body. Due to aortic stenosis, the heart pumps with greater force, which increases the pressure in the heart. This increase in pressure is reflected in the lungs. In this particular patient, this puts the transplanted lungs at risk of deterioration.
The TAVR procedure was successfully performed in the catheterization laboratory under conscious sedation by Dr Ravinder Singh Rao and the team at Breach Candy Hospital, Mumbai. The femoral access was obtained under ultrasound guidance. A small catheter was inserted in the femoral (groin) blood vessel. It was advanced to the heart. The new valve was positioned correctly and deployed inside the old valve. The new valve started functioning immediately. Immediately, the pressure inside the heart chambers returned to normal; hence, the pressure inside the lungs also normalised. There were no TAVR-related complications like pacemaker, stroke or leakage. There was no cut involved. A small endoscopic suture was placed inside the artery, and the entry point was sealed by closing the suture.
The patient was immediately shifted to the cardiac care unit (CCU) for observation, and she could sleep in a supine (flat) position, and by the next day, she was able to walk without breathlessness. A quick recovery for a patient of this status is rare since the life expectancy of lung transplant patients is shorter than that of other organ transplants. This can be credited to the high-calibre quality of the transplant expert, Dr Sandeep Attawar, and Dr Ravinder Singh Rao, who has the highest experience in TAVR procedures with safe outcomes in the country.
Dr. Ravinder Singh Rao, Interventional Structural Cardiologist and TAVR expert, remarked, “To our knowledge, this is the first case of successful TAVR in a lung transplant recipient. Lung transplant patients are often at high risk for open heart surgery due to an immunosuppressive regimen that has to be followed post-transplantation. Considering their severely reduced cardiac output, TAVR remains the only feasible option for such patients with severe aortic stenosis, especially when open heart surgery has been declined. TAVR has to be done safely in such patients as there is no room for error. TAVR benefits these patients as the symptoms are improved significantly and the patient’s quality of life is restored before aortic stenosis becomes a life-threatening condition.”
The patient was discharged two days later in good clinical condition. On discharge, the transthoracic echocardiogram showed a well-functioning aortic valve and normal functioning of the heart. The team consisted of Dr Ravinder Singh Rao (Interventional Structural Cardiologist, TAVR and Mitraclip expert), Dr Nimit Shah (Interventional Cardiologist), Dr Kalpana Shah (Cardiac Anaesthetist), and Dr Kamlesh Jain (Cardiac Surgeon).
A patient’s family also plays a very important role in such illnesses—constant support from the spouse, motivation, and active participation in disease management are required. The patient and their family become an integral part of the medical team in such complex diseases.
2, Aug 2024
Apollo Cancer Centre Doctors Use Robotic Surgery to Remove 1.13 kg Adrenal Tumour, Giving Middle-Aged Man New Lease on Life
Chennai, 2nd August 2024: Apollo Cancer Centre, one of the top super speciality hospitals in India, performed a robot-assisted surgery to remove an extremely large adrenal myelolipoma (a non-cancerous tumour of the adrenal gland) measuring 18 cm and weighing over a kilogram to save a 49-year-old male patient. This complex robot-assisted surgery was performed by Dr. Ragavan N, Senior Consultant in Uro-Oncology, and his team from Apollo Cancer Centre using the da Vinci Xi robotic platform by Intuitive.

The patient was diagnosed with a large adrenal tumour, 18 cm in size, significantly larger than the average tumour, which typically measures 5 to 7 cm. Unlike standard cases, where an adrenal tumour sits on top of the kidney, this tumour encroached behind the right kidney, compressing the IVC (Inferior Vena Cava), as well as a significant part of the liver. The pre-operative workup indicated that the tumour was likely a myelolipoma. The doctors at Apollo Cancer Centre decided to proceed with robot-assisted adrenalectomy due to the significant risks posed by the size and position of the tumour.
Commenting on the complexity of this case, Dr. Ragavan N, Senior Consultant in Uro-Oncology at Apollo Cancer Centre, said, “In open surgery, handling such a large tumour can pose significant challenges. The soft and fragile nature of adrenal tumours, especially myelolipoma, which is composed of fat cells, can increase the risk of rupturing the tumour during manipulation. Even though the pre-operative evaluation suggested a myelolipoma, we were concerned about the probability of malignancy. Any rupture of the tumour (especially if it was malignant) could have led to metastasis (spread of cancer cells). Therefore, the decision to perform robotic-assisted surgery with the da Vinci system was crucial because of the dexterity, precision, and stability offered by the robotic arms, which enhanced our ability to lift and remove the tumour safely without causing any rupture.”
Expressing his gratitude, the 49-year-old patient said, “I’m extremely thankful to Dr. Ragavan N and the medical team at Apollo Cancer Centre who assisted him during the surgery. They counselled me about the benefits and risks of undergoing robot-assisted surgery and executed the surgery to remove the tumour safely without any complications. Without their support and care, I would not have recovered so quickly and returned to my normal life sooner than I expected.”
Dr. Ragavan N further elaborated, “Considering how obese the patient was (110 kg), the complex position and large size of the tumour, open surgery would have posed immense challenges. Removing such a large tumour would have required a 25 – 30 cm incision, which would have significantly impacted wound healing and recovery time, increasing the risk of developing a hernia. Locating the adrenal vein, a very critical yet extremely small structure, would have been extremely difficult in this case owing to the enormous size of the tumour. The minimally invasive nature of robot-assisted surgery with its 3D vision and dexterity made it easier for us to navigate through the challenges and perform the surgery. The enhanced precision and control offered by robot-assisted surgery minimize the risk and improve patient outcomes.”
1, Aug 2024
AccuHealth Chairman Vows Top-Tier CT Scan & More Services

Aug 01, 2024,Baruipur, West Bengal, India : AccuHealth Diagnostics is excited to announce the launch of its advanced CT Scan facility at the Baruipur branch. This new addition brings high-quality, accessible healthcare to the residents of Baruipur and surrounding areas, ensuring state-of-the-art diagnostic services right at their doorstep.
The new 32-slice CT Scan machine from GE Healthcare provides detailed and accurate imaging, rapid scanning, and 40% less radiation. This means safer and quicker scans for patients. With superior imaging quality and total patient comfort, the CT Scan at AccuHealth Baruipur is designed with patients’ well-being in mind. The machine also features a short breath hold capability, making it ideal for patients with breathing issues, elderly patients, and children.
Message from the Chairman
Mr. Anirban Das, Chairman of AccuHealth, expressed his company’s dedication and commitment to bringing world-class healthcare closer to home.
He said, “AccuHealth is forever committed to the health and wellness of the community. We are thrilled to introduce our advanced CT Scan to AccuHealth Baruipur. We are confident this will benefit the lives of patients in this area – they won’t need to travel far, wait in long queues or overspend for top-tier medical imaging.”
He also emphasized AccuHealth’s range of diagnostic services, including advanced pediatric echocardiography and oncology services for newborn babies, ultrasound, colour Doppler, and Holter monitoring. “Our polyclinic is staffed by a highly qualified team of reputed doctors, providing comprehensive healthcare under one roof,” he added.
Special Pediatric Echocardiography Service
Another feather on AccuHealth’s cap is the first pediatric echocardiography service in Baruipur and South 24 Parganas. This specialised service is designed to cater to the unique cardiac needs of children, providing accurate and timely diagnoses to support better health outcomes.
The clinic features state-of-the-art high-resolution echocardiography machines that deliver exceptionally clear and detailed images of the heart. With advanced 3D and 4D imaging techniques, patients benefit from dynamic and comprehensive heart views, making it easier for doctors to diagnose and plan treatments accurately.
Patients at AccuHealth can feel confident in the team’s expertise, which includes certified radiologists, cardiologists and sonographers. This ensures that every examination is performed with the highest level of precision.
Top-Notch Gastroenterology Services
AccuHealth Baruipur also provides a full range of gastroenterology services designed to ensure comprehensive care. Patients can undergo important tests like colonoscopy to check for conditions in the colon and rectum, upper GI endoscopy to examine the esophagus, stomach, and duodenum, and flexible sigmoidoscopy for the lower colon. For liver assessments, a liver biopsy is also available when needed, making sure every aspect of gastrointestinal health is covered.
The facility prioritises patient comfort throughout the process. The examination and procedure rooms are designed to be comfortable and inviting. Patients receive clear and supportive instructions for preparation, including any necessary dietary changes. After procedures, patients can relax in designated recovery areas and get detailed follow-up instructions to help them feel their best and recover smoothly.
Building a Legacy Trust and Excellence
Over the years, AccuHealth has earned a solid reputation in Baruipur and surrounding areas by consistently delivering high-quality diagnostic services. The diagnostic chain’s commitment to excellence is reflected in the positive reviews it has received and its dedication to maintaining this trust by continuously enhancing its services.
30, Jul 2024
Watermelon-Sized Uterine Tumor Weighing 2.2 Kgs Successfully Removed from 49-Year-Old Woman via Robot-Aided Surgery at Fortis Noida
Noida, 30th July 2024: Doctors at Fortis Hospital, Noida successfully operated and removed a watermelon sized non-cancerous tumor weighing 2.2 kgs from the uterus of a 49-year-old woman via Robot-aided surgery. The team of doctors led by Dr Anjana Singh, Director & HOD, Obstetrics and Gynaecology, Fortis Hospital, Noida conducted the complex surgery, which lasted for 3 hours. The patient was discharged in stable condition two days later.
Upon admission at Fortis Noida the patient complained of severe pain during her menstruation cycle, along with irregular and heavy bleeding for almost 2 years. An ultrasound revealed a large fibroid (tumour) measuring 17-18 cms in size along with other fibroids of 7-8 cm. Such fibroids typically form when the body secretes excessive estrogen, which causes the growth of fibroid tissues. The large mass of tumour had occupied the entire abdominal area, thus making it extremely difficult to operate. Additionally, the patient had undergone two prior C-sections, and her scars were vertical. In such cases, there is always a risk of injury to the vital organs during dissection of the adhesions. Hence, Robot-assisted surgery was adopted to better identify the anatomical structures and minimize the risk of unintended damage to the internal organs.

Giving details of the case, Dr Anjana Singh, Director & HOD, Obstetrics and Gynaecology, Fortis Hospital, Noida said, “The patient had travelled from Aligarh, as there she was advised to undergo traditional open surgery by multiple gynaecologists, which the patient did not want to opt for. That’s when she came to Fortis. In this case, the tumour had grown to the sizeof a watermelon. We had to be extremely careful while operating to save the vital organs, and that’s where robot-assisted surgery provided enhanced precision. The surgery involved very little intraoperative bleeding with no requirement for blood transfusion, owing to high precision. The most challenging part of this surgery was to retrieve such heavy tumour from 8 mms miniature incisions on abdomen used for the robotic arms and camera. We decided to break down the tumour in a bag inside the abdomen followed by removal of small pieces of fibroid from the very small incision. Had the surgery not been performed on time, the fibroid would have grown immensely in size and would have put pressure on surrounding organs such as bowel and bladder, causing intestinal obstruction and urine retention. Bleeding and pain during menstruation cycle would also have increased and excessive bleeding could have made the patient severely anaemic. Such cases are rare as tumours weighing 2.2 kgs are found in only 10-20% cases and in 1% of such cases, a massively growing fibroid can become malignant.”
Mohit Singh, Zonal Director, Fortis Hospital Noida said, “This was a very critical and challenging case considering the size and weight of the tumour, along with two c-section surgeries in the past. Despite the challenges, the surgery was successfully conducted owing to the correct medical assessment and the patient was able to resume her daily routine in just one week of surgery. Clinical expertise and best-in-class care to manage such cases are the hallmarks of Fortis Hospital Noida, and we continuously endeavor to provide highest level of care to save lives and get improved outcomes.”
29, Jul 2024
Merck Foundation Declared Botswana First Lady as Ambassador of More Than a Mother

July 29, 2024,Gaborone, Botswana & Mumbai, Maharashtra, India : Merck Foundation, the philanthropic arm of Merck KGaA Germany officially launched their programs in partnership with H.E. Mrs. NEO JANE MASISI, The First Lady of Botswana, also the Ambassador of “Merck Foundation More Than a Mother”, the programs which started in 2018 with the aim to transform patient care, build healthcare capacity, break the stigma of infertility, empower women, support girl education, stop GBV in Botswana and the rest of Africa.
The program took place in esteemed presence of The President of the Republic of Botswana, H.E. Dr. MOKGWEETSI ERIC KEABETSWE MASISI and Chairman of Merck Foundation Board of Trustees, Prof. Dr. Frank Stangenberg-Haverkamp.
The Summit was chaired by The First Lady of Botswana, H.E. Mrs. NEO JANE MASISI, and CEO of Merck Foundation, Senator, Dr. Rasha Kelej.
H.E. Dr. MOKGWEETSI ERIC MASISI, the President of Botswana emphasized during the launch “I deeply appreciate the joint programs of The First Lady of Botswana and the Merck Foundation. I am thrilled to see the tangible, measurable impact of these programs and their long-term commitment to the health and social development of our beautiful country, in such a short time. Botswana needs these valuable programs; I wholeheartedly wish for their success.”
Senator, Dr. Rasha Kelej, CEO of Merck Foundation and President of “More Than a Mother” Campaign emphasized, “It is a great honor to meet H.E. The President of Republic of Botswana, and our long-term partner and my dear sister, H.E. Mrs. NEO JANE MASISI, The First Lady of Republic of Botswana and Ambassador of “Merck Foundation More Than a Mother” to officially launch the Merck Foundation Programs in the country, and to underscore our commitment towards building healthcare and media capacity, patient care landscape transformation, ending GBV, breaking infertility stigma and supporting girl education, together in the country.”
H.E. Mrs. NEO JANE MASISI, The First Lady of Republic of Botswana, Ambassador of “Merck Foundation More Than a Mother” expressed, “It is a pleasure to welcome and meet Merck Foundation Chairman and CEO to our country. Together we officially launched and also celebrated important milestones of the great success of our joint programs to build healthcare capacity, transform patientcare, break infertility stigma, support girl education and stop GBV, in our country since 2018.
In a very short time, we have been able to provide 46 scholarships to our young doctors in many specialties which are very critical for us. We are proud of our achievements together.”
“I am very happy to share that we have together transformed the patientcare landscape of Botswana by providing 46 scholarships of One-Year Online PG Diploma and Two-Year Master Degree for local doctors from Botswana nationwide in many underserved and critical medical specialties such as Oncology, Diabetes, Endocrinology, Embryology, Fertility, Sexual and Reproductive care, Acute Medicine, Dermatology, Obesity and Weight Management, Respiratory Care and Internal Medicine and more, it is considered as an excellent number for 2.7 M population. And we are still going to provide more scholarships to local healthcare providers nationwide,” emphasized Prof. Dr. Frank Stangenberg Haverkamp, Merck Foundation Board of Trustees.
“I am also very happy to share that together with Her Excellency The First Lady of Botswana, we are also supporting girl education by providing scholarships and bicycles for 40 schoolgirls to cover their transportation and other education expenses so that they can reach their potentials and achieve their dreams,” Dr. Rasha Kelej added.
Prof. Dr. Frank Stangenberg Haverkamp added, “Our aim is to improve the overall health and wellbeing of people by building healthcare capacity across Africa, Asia and other developing countries. We are strongly committed to transforming patientcare landscape through our scholarships program.”
“Merck Foundation has provided 1810 scholarships to doctors from 52 countries in 42 critical and underserved medical specialties. Out of the total 46 scholarships provided in Botswana, 19 scholarships have been provided for Diabetes, Endocrinology, and Obesity & Weight Management, for Botswanan doctors from different provinces across the country, which is very important to improve access to quality and equitable diabetes and hypertension patient care not only in Gaborone but nationwide. After completion of the course, these doctors will be able to establish diabetes or hypertension clinics in their Health Centre or Hospital with the aim to help prevent and manage the disease in their own communities,” Senator, Dr. Rasha Kelej added.
Out of the 46 scholarships, 10 scholarships have been provided to doctors in Fertility and one-year PG Diploma and two-year Master degree in Sexual and Reproductive Medicine. This is a huge number that will advance women’s health in the country.
5 Scholarships have been provided for Oncology to develop and support the cancer care capacity in the country.
Moreover, 12 scholarships have been provided for Acute Medicine, Respiratory Care, Dermatology, Gastroenterology and Internal Medicine and more as part of Merck Foundation Capacity Advancement Program.
Merck Foundation is also working closely with Botswana First Lady to inspire girls and sensitize communities about the importance of girl education. As a part of their ‘Educating Linda’ program, Merck Foundation will sponsor the education of 20 deserving schoolgirls, till they graduate. Moreover, Merck Foundation also provided 20 bicycles to encourage girls to continue going to the school, as they sometimes dropout due to long distances.
“I really believe that when the girls are educated, their countries become more powerful, stronger & prosperous,” added Senator Kelej.
Merck Foundation Chairman and CEO together with The First Lady of Botswana also visited schools in the country to meet the beneficiaries of Sewing machines donation and witness their success.
During the Summit, Merck Foundation Awards Ceremony was held, during which 7 Winners of the Merck Foundation Awards were acknowledged by Merck Foundation Chairman, Merck Foundation CEO together with Botswana First Lady.
Additionally, they also addressed the journalists during the Merck Foundation Health Media Training conducted in partnership with The First Lady of Botswana for the Botswanan journalists, to emphasize on the important role that media plays to influence society to create a cultural shift with the aim to address wide range of social and health issues such as: Breaking Infertility Stigma, Supporting Girl Education, Women Empowerment, Ending Child Marriage, Ending FGM, Stopping GBV, Diabetes and Hypertension awareness. Apart from this, the Call for Application for 8 important Merck Foundation Awards in partnership with The First Lady of Botswana were announced for Media, Musicians, Fashion Designers, Filmmakers, students, and new potential talents in these fields Merck Foundation. The training program was also addressed by prominent Medical & Media Experts and Government Officials.
Moreover, Merck Foundation Chairman, and Merck Foundation CEO together with The First Lady of Botswana signed a few copies of their different children’s storybooks titled: “More Than a Mother” created for children and youth to emphasize and strengthen family values of love and respect from an early age; “Not Who You Are” to teach boys to love and respect their future wives and eliminate domestic violence, to emphasize on the importance of empowering girls through education and “Sugar Free Jude” and “Mark’s Pressure” to promote a healthy lifestyle and raise awareness on the early detection and prevention of Diabetes & Hypertension. Thousands of copies of these storybooks are going to be distributed to school students of Botswana.
26, Jul 2024
Clinical Studies Highlight the Potential of Tesla BioHealing Biophoton Generator in Improving Mobility for Chronic Stroke Patients

Butler, PA, July 26, 2024 — First Institutes of All Medicines conducted randomized, double-blind and placebo-controlled clinical study to determine effectiveness of the Tesla BioHealing Biophoton Generator when used by 42 chronic stroke patients.
The newly completed clinical studies indicate that the Tesla BioHealing® Biophoton Generator may help enhance mobility in patients with chronic stroke conditions. These outcomes represent a significant step forward in addressing permanent disability caused by strokes. Initial small-scale studies provided sufficient evidence to justify a larger confirmatory clinical study.
Led by Dr. Mariola Smotrys at the First Institute of All Medicines, a randomized, double-blind and placebo-controlled clinical study was conducted in 42 patients with chronic stroke. The majority of patients treated with the Tesla BioHealing® Biophoton Generator experienced improvements in mobility and quality of life. The results suggest that this technology could provide new hope for many stroke survivors.
Dr. Lawrence Alpert, the study physician, shared his positive perspective on the findings. “The Tesla BioHealing® Biophoton Generator, currently available as a wellness product, has shown promise in stroke rehabilitation,” said Alpert. “Clinical trials have demonstrated that this device can significantly improve the quality of life for chronic stroke patients, offering them a chance to regain independence and mobility.”
The Tesla BioHealing® Biophoton Generator emits ultra-high biophotons that support cellular repair, regeneration and communication. This non-invasive treatment works with the body’s natural healing processes, providing a safe and potentially effective solution for stroke patients who have not benefited from conventional rehabilitation methods after six months.
“I experienced significant improvements after using the Tesla Biophoton Generator,” said Les Duncan, a 74-year-old six-time stroke survivor. “My pain and brain fog have cleared, and I no longer need a walker. I feel like a new person.”
Dr. James Liu, CEO of Tesla BioHealing, Inc. stated that the new results of the clinical studies encourage our organization to develop a safe, effective and easy to use product to benefit patients. We are to prepare and apply regulatory approval. Our goal is to advance this innovative technology to benefit millions of patients with chronic stroke.
Forward-Looking Statements
This press release contains forward-looking statements that involve certain risks and uncertainties. These statements include, but are not limited to, potential benefits of Tesla BioHealing® products. Factors that could cause actual results to differ materially include risks that clinical development activities may be delayed or unsuccessful, that products may not be approved or commercially viable and that regulatory decisions may be unfavorable. Tesla BioHealing Inc. and the First Institute of All Medicines (FIAM) disclaim any obligation to update these statements after the date hereof except as required by law.
25, Jul 2024
Eisai Selects Medidata’s Clinical Data Studio to Enhance and Modernize Clinical Trial Efficiency and Patient Experience

July 25, 2024,New York, United States : Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced Eisai Inc. (“Eisai”), the U.S. pharmaceutical subsidiary of Tokyo-based Eisai Co., Ltd., as one of the first customers to harness its recently announced AI-driven Medidata Clinical Data Studio. Eisai Inc., will leverage this innovative data experience to gain unprecedented control over its clinical data, enable the execution of scalable and complex clinical trials, and enhance patient experience.
“We’ve included Medidata’s Clinical Data Studio in our clinical trial management platform given its ability to break down data silos and seamlessly integrate into our current software stack, while maintaining quality and integrity across all data sources,” said Shobha Dhadda, Ph.D. chief clinical science & operations officer, at Eisai. “Having a suite of technology solutions capable of processing diverse clinical and patient data types provides increased efficiencies without sacrificing quality or needing additional resources.”
Clinical Data Studio is powered by the Medidata Platform, the industry’s only unified platform that centrally manages all data sources, improving data reliability across the entire clinical trial ecosystem. By seamlessly integrating data from both Medidata sources, including Medidata Rave EDC, and non-Medidata sources, such as labs or another electronic data capture system, Clinical Data Studio streamlines the import process and enables automatic validation through configured data transfer agreements. Utilizing AI, it mitigates challenges posed by disparate data systems and offers up to 80 percent faster data review while providing a comprehensive view of patient data that can be concurrently reviewed, visualized, and acted on.
“Through Clinical Data Studio, Eisai is enabling healthcare stakeholders to overcome the complexities of modern clinical trials and foster collaboration on cleaner, more actionable data,” said Janet Butler, executive vice president, global head of sales, Medidata. “By delivering a unified AI-driven data management and analytics experience, we are enabling study teams to identify potential data issues faster and gain a more accurate understanding of the patient.”
24, Jul 2024
BD India’s Concerted Commitment Towards Creating Awareness About Venous Diseases

24th July 2024 New Delhi, Delhi, India To create awareness and enable knowledge-sharing around Venous diseases, in order to drive better patient care BD (Becton, Dickinson, and Company) conducted roadshows across New Delhi, Mumbai and Bangalore recently. Venous disease is a condition where the veins in the body have difficulty returning blood to the heart, thereby resulting in blood clot forming in a vein, typically in the legs. If left untreated, these clots can break loose and travel to the lungs, causing serious complications. Delayed detection and intervention can lead to worsened clinical outcomes and a multitude of problems for both patients and healthcare professionals.
The 3-day Venous Summit witnessed a series of Technology Innovation Forums followed by Symposiums driven by Dr. Houman Jalaie, Head of Venous Commission of German Society of Vascular Surgery and Deputy Head of Department of Vascular & Endovascular Surgery, University Hospital Aachen, Germany and over 40 prominent faculty from Interventional Radiology, Vascular Surgery from ~12 cities across India. Attended by 200+ delegates with 13 case-presenters who drove 8.5 hours of scientific discussions each day, the end objective of the Summit was to discuss best domestic and international practices in order to drive better patient care in the venous domain in India.
Commenting on this initiative to establish Standard of care for Venous disease patients in India, Atul Grover, Managing Director, India, South Asia, “Venous Diseases are slow progressive diseases which exist very commonly but often go untreated owing to low levels of awareness and lack of diagnosis. Through our Venous Summit, in partnership with experts like Dr. Houman Jalaie we are bringing the focus on Venous ailments as well as providing a platform to healthcare experts from across the country to establish a uniform quality care protocol to enable better patient care. This initiative is aligned with BD’s purpose of advancing the world of health by delivering superior patient outcomes.”
Dr. Houman Jalaie shared his knowledge with healthcare professionals through a series of events which touched upon the following areas:
- Uniform treatment algorithms in Post-Thrombotic Syndrome and NIVL management
- Approaches to learn and adapt from current International and National best practices in Deep Vein Thrombosis (DVT) Management
- How novel technologies, like mechanical thrombectomy, can improve the patient outcomes in complex venous diseases
Reflecting on his experience, Dr. Jalaie said, “As a healthcare professional, there is nothing more important for me than the safety and wellbeing of my patients. I was delighted to interact with so many like-minded senior doctors from all over India to discuss the right strategies to enable better patient outcomes.”
Every year over 2 million patients in India suffer from some form of Venous disease, however, due to less awareness, many patients go undiagnosed or untreated.